The Skinny
Average Recovery
1 days
Permanence
Temporary
Application
Injected
Surgical
No
Cost
$100 - $1498
AEDIT
Before & After Images by Provider



Before & After Images by Provider
Xeomin®
The Specifics
What is Xeomin®?
Xeomin®, by Merz, is primarily composed of the active ingredient incobotulinumtoxinA. Unlike Botox®, Dysport®, and Jeuveau®, Xeomin® does not combine botulinum toxin type A (BoNT-A) with complex protective proteins. It does contain inactive ingredients like human albumin and sucrose similarly to Botox®, Dysport, and Jeuveau®.
Where to Use Neurotoxin vs. Filler
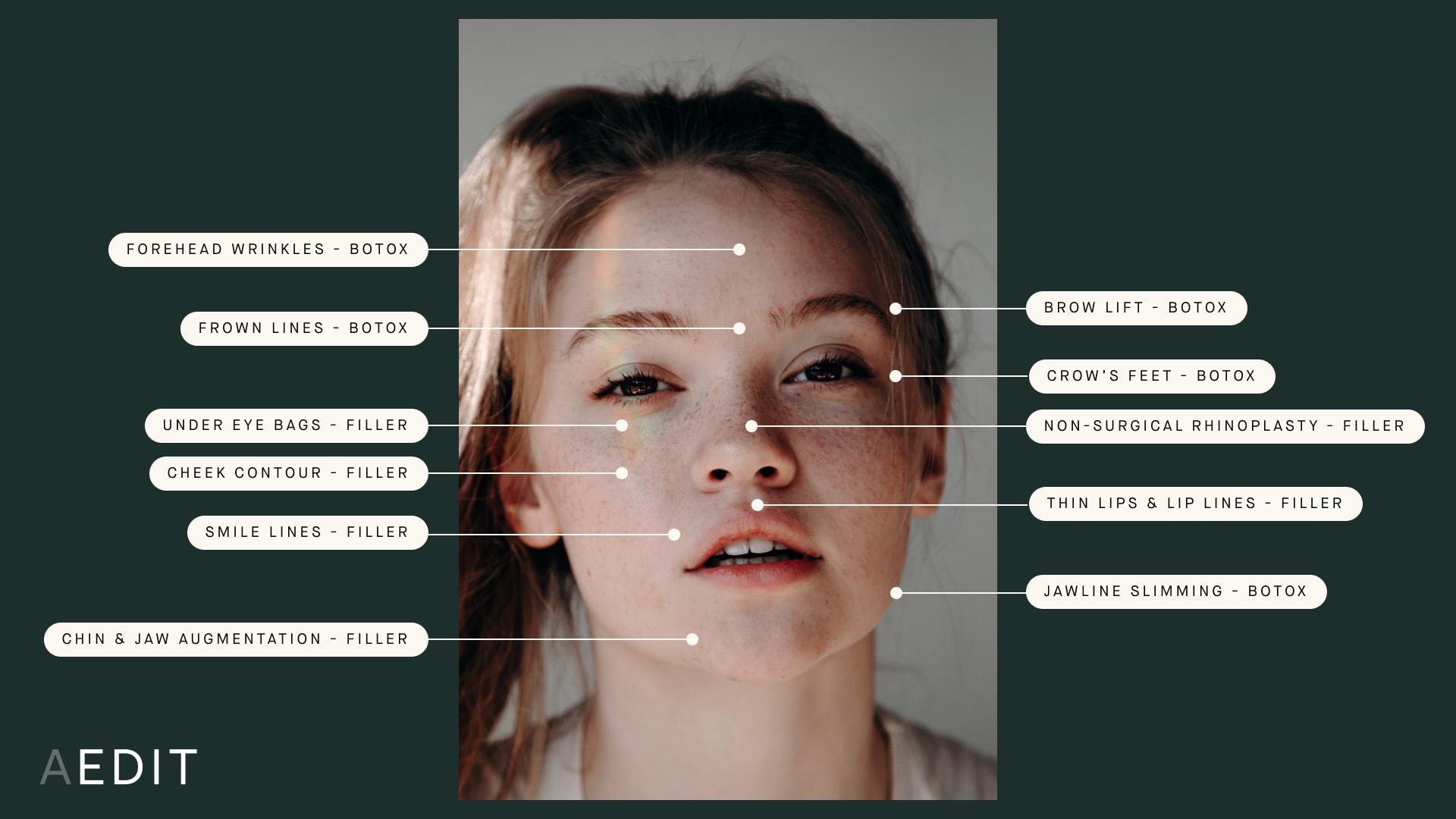
Due to the lack of protective proteins, Xeomin® does not have to be refrigerated, which may make injections more comfortable due to the higher temperature of the formulation. Individuals experiencing an allergic reaction to Botox® and Dysport® may tolerate Xeomin®. Xeomin® is United States Food and Drug Administration (FDA) approved to treat frown lines, muscle spasticity, and excessive drooling.
BoNT-A (and all of its specially formulated offshoots like Botox®, Dysport®, and Jueveau®) functions by inhibiting cell transmissions between nerve and muscle cells creating a flaccid paralysis. The results of the injectable are temporary and the body will naturally break down the incobotulinumtoxinA (Xeomin®) over time.
What cosmetic concerns does Xeomin® treat?
Who is the ideal candidate for Xeomin®?
The ideal candidate for Xeomin® injections is seeking a tried, safe, and efficacious temporary solution to common cosmetic concerns. Xeomin® may be a slightly more comfortable procedure than Botox®, Dysport®, and Jeaveau® due to the lack of refrigeration.
Xeomin® is not recommended for those under 18, women who are pregnant or nursing, anyone with an allergy to the components of Xeomin®, or those with certain chronic medical conditions.
What is the average recovery associated with Xeomin®?
Most candidates experience minimal side effects from a Xeomin® injectable lasting no more than one day. Mild swelling, pain, and redness at the injection site resolve almost immediately following the injections. Many patients resume normal activities following the Xeomin® injection.
To better understand the healing and downtime associated with the procedure, check out our complete guide to neurotoxin recovery.
What are the potential side effects of Xeomin®?
Mild side effects of Xeomin® are very similar to Botox®, Dysport®, and Jeaveau® and include skin changes like redness, dryness, and flaking. Warmth, itchiness, tightness, injection site infection, and/or bruising may occur. It is also not uncommon to experience dizziness, weakness, headache, or vision problems. These side effects usually resolve within one to two days.
More serious, uncommon side effects of Xeomin® include double vision, muscle spasms, severe muscle weakness, hoarseness, and/or trouble breathing/wheezing. These more serious side effects typically resolve within three to four months. Severe life threatening allergic reactions can occur.
What can someone expect from the results of Xeomin®?
Xeomin® results are temporary, and become noticeable after three to five days. Full results are usually visible within two weeks. Maintenance injections of Xeomin® will most likely be needed every three to four months depending on the individual and the treatment.
What is the average cost of Xeomin®?
The average cost of Xeomin® is $100 to $1,500. The actual cost of the Xeomin® injection will vary by procedure, location, healthcare provider, and individual candidate needs. Learn more in our complete guide to Xeomin® cost.
Pros
- Minimally Invasive
- No Downtime
- Lower Initial Costs
Cons
- Temporary Results
- Reoccurring Expense
Invasiveness Score
Invasiveness is graded based on factors such as anesthesia practices, incisions, and recovery notes common to this procedure.
What to Expect
Xeomin® injections are a simple, outpatient procedure with few side effects and short recovery. Here is a general guide for what to expect before, during, and after a Xeomin® injection: